What is the difference between natural and artificial competent cells?
Competent cells can be naturally occurring or cells can be artificially made competent.
The competence of natural cells is genetically determined, that is, a bacterium is genetically predisposed to take up free (genetically competent) genetic material that exists in its environment. Research has shown that competent bacterial cells can take up DNA from a variety of sources; however, some bacteria show specificity or preference for DNA fragments that are overrepresented within their genome. DNA taken up by a naturally competent cell is not always incorporated into the cell’s genome. Often a piece of DNA will be used for nutritional purposes.
For example, DNA provides the cell with a much-needed source of deoxyribonucleotides for replication. Determining how DNA is used within a cell generally depends on the needs of the cell. Other factors include existing DNA damage within the cell and the ability of incoming DNA to recombine. Although a cell can be considered naturally competent, the state of competition is not necessarily a constant state. In fact, the natural regulation of competition and transformation is important to protect a cell. Regulation often occurs through environmental and biochemical signals.
For example, Streptococcus pneumoniae is a naturally competent bacteria; however, their competition is driven by quorum sensing, or sensing and responding to cell population density through gene expression. For natural transformation to occur, there must be donor DNA present in the environment. One study found that when conditions were met, the donor DNA came from a “subfraction” of the S. pneumoniae population, while the other part was competent and took up the DNA.
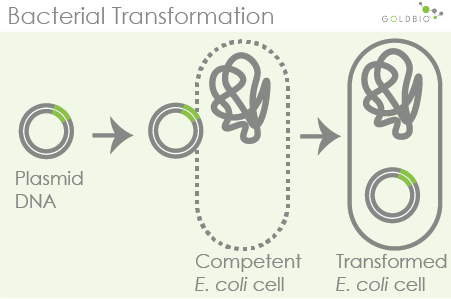
Artificial or induced competent cells are cells that researchers have made competent by electrical (electroporation) or chemical manipulation. Cell competition has become an essential research tool for cloning because it provides scientists with a mechanism to introduce new genetic material into a cell. When we think of competent cells in a research setting, this is often the type of cellular competition that we mean. While there are many naturally occurring competent cells, the model organism E. coli is not naturally competent. Therefore, in order to use these cells for cloning, researchers must induce competition.
Whether through electroporation or chemical methods such as calcium chloride, the process of creating competent cells creates temporary pores in a cell’s membrane for DNA to pass through. Remember, competition is a cell’s ability to absorb foreign DNA from its environment, while transformation is the actual process of DNA absorption. Therefore, for a scientist to transform a cell, he must first make that cell competent. This is done by changing the cell in a way that allows the DNA to easily travel through the cell membrane.
What exactly makes a cell competent?
Since natural competition is genetically determined, these cells will be equipped with special machinery to facilitate natural transformation and will often have biochemical and environmental cues to regulate competition. In a laboratory setting, usually with E. coli, artificial cell competition is possible through a chemical process or through electroporation. Both methods disrupt the cell membrane, creating temporary pores that allow DNA to enter the cell.
Artificially Competent Cell Types
There are two types of artificially competent cells: chemically competent and electrocompetent.
1. Chemically competent cells
Chemically competent cells are cells that were made competent with a salt treatment followed by a heat shock step. This process permeabilizes the cell membrane, allowing the entry of the plasmid. Protocols using CaCl2 or MgCl2 are the most common method of making chemically competent cells, but some protocols involve other salts or combinations of various salts and chemicals.
List of salts and chemicals used to make chemically competent cells
What role does CaCl2 and heat shock treatment play in creating competent cells?
When making chemically competent cells, the first step involves the use of a salt, usually CaCl2 or MgCl2. Salt (chemical) treatment neutralizes the negative charges of the phosphate heads and negatively charged DNA. Neutralizing these charges removes the natural repulsion, allowing the DNA to move closer to the cell.
The heat-shock step involves rapidly cooling and heating the cells, leading to temporary pores that allow DNA to enter the cell. The mechanism behind how this works is not really well understood. One study suggests that the passage of the heat pulse causes E. coli to release lipids from its surface, which generates temporary pores that allow DNA to enter the cell.
2. Electrocompetent cells
Electrocompetent cells are made competent by using an electrical pulse from an electroporator to create temporary pores (poration) in the cell membrane of prokaryotic or eukaryotic cells. The electrical pulse ruptures the cell membrane, causing a slight realignment of the lipid bilayer, allowing exogenous material to enter the cell. Although exogenous material can enter the cell due to increased permeability, cellular material can also be lost during the process.
Researchers choose to use electroporation for many different reasons. The higher transformation efficiency of this method compared to other methods is one of the reasons for the choice. Another reason researchers might use electroporation is to introduce other materials, such as new drugs or molecular probes, into the cell.
How does electroporation work?
Phospholipid bilayers are dual chains of phospholipids (phospholipids are composed of a hydrophilic head and a hydrophobic tail). The hydrophilic heads of the bilayer face outward: one row faces the extracellular space, while the other row faces the intracellular fluid. The hydrophobic tails face each other, that is, the inner portion of the bilayer. When electroporation voltage is applied, the electrical pulse rearranges the orientation of the bilayer so that it forms a gap.
The difference between reversible and irreversible electroporation.
Reversible electroporation (RE) involves a voltage of up to 1 kV. Cells that undergo reversible electroporation can survive the effects of membrane penetration and recover (the cell membrane can later reseal). Molecular biologists use this technique to temporarily penetrate the cell membrane in order to introduce foreign molecular material, such as DNA, into a cell.
Irreversible electroporation (IRE) involves up to 3 kV of direct current. Unlike reversible electroporation where the membrane nanopores are temporary, irreversible electroporation creates permanent pores that ultimately lead to apoptosis (programmed cell death). Researchers have been using IRE to treat tumours. Therefore, IRE is not going to be a method used for molecular transformation and transfection.